orange book pharmacy definition
Most pharmacists already know that the Orange Book created in 1980 and now in its 28th edition is an FDA publication that lists many drug products and contains indications as to whether generic versions of medications are considered to be equivalent to the drugs manufactured by the innovator company and most often marketed with brand names. Rucha Pathak Roll No.
Before understanding different drug ratings it is necessary.

. An introduction a how to use section the drug product lists appendices and a patent and exclusivity information addendum. Updated with Orange Book. The publication Approved Drug Products With Therapeutic Equivalence Evaluations the List commonly known as the Orange Book identifies drug products approved on the basis of safety and.
The FDA keeps a list known as the Orange Book of every approved therapeutic equivalent. FDA orange book The official name of FDAs orange book is Approved Drug Products with Therapeutic Equivalence Evaluations. The orange book is a list of generic drugs approved by FDA.
Formally known as Approved Drug Products with Therapeutic Equivalence Evaluations the orange book lists drugs which are not only safe but also effective for human use. One prescription example would be combined oral contraception also. Reference Standard RS A reference standard is the drug product selected by FDA that an applicant seeking approval.
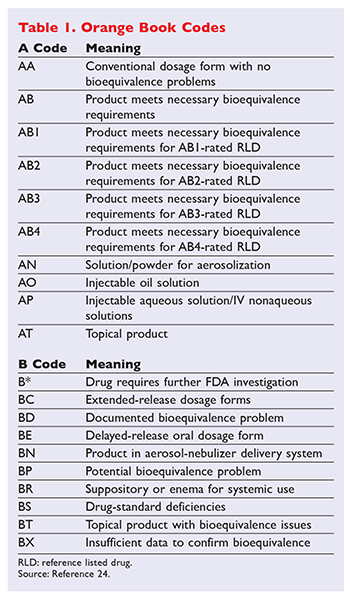
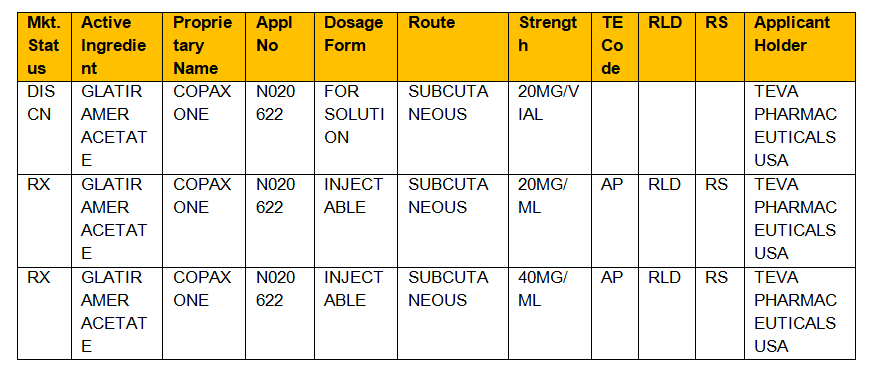
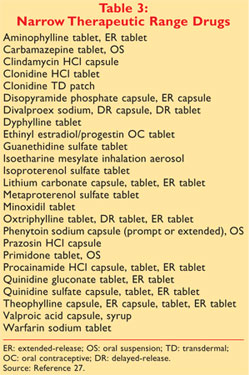
Originally this book was published in October 1980 with orange cover and thus the name orange book. Administration Orange Book provided however that drug products found by the United States Food and Drug Administration to have a narrow therapeutic range shall not be considered generically equivalent for the purposes of this act. In the electronic Orange Book an RLD is identified by RLD in the RLD column.
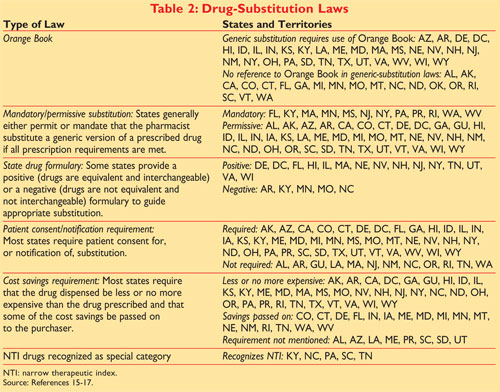
The full publication title is Approved Drug Products with Therapeutic Equivalence Evaluations but it is commonly known as the Orange Book1 Generic substitution laws are state specific and many require use of the Orange. Approved Drug Products with Therapeutic Equivalence Evaluations. The Orange book has been revised.
The Orange Book is an important publication published by the FDA that serves as the gold standard reference for generic drug substitution. The orange book is published annually and the 2015 edition is 35th edition of orange book1 It is freely available for. Basics in drug approval process with reference to the Orange Book Presented by.
The orange book consist of five main sections. Get emails about this page.
Insights Into Effective Generic Substitution
Regulatory 101 Drug Name Modifiers Definition Categories Generics And Capa Ivt

Therapeutic Equivalence Definition Examples Video Lesson Transcript Study Com
/color-therapy-definition-types-techniques-and-efficacy-5194910_final1-972d594f507449908c885a41347a4a1d.png)
Color Therapy Definition Types Techniques Efficacy

Appendix D Understanding Drug Information Drug Names And Pronunciations In Manual For Pharmacy Technicians
Pharmacy June 2021 Browse Articles

Innate Vs Adaptive Immunity Immunology Pharmacy Books Immunity

Regulatory 101 Drug Name Modifiers Definition Categories Generics And Capa Ivt
Orange Book And Its Applications Legal Advantage
The Hatch Waxman Act 25 Years Later Keeping The Pharmaceutical Scales Balanced

Mcat Cars Practice Question From Mcat Prep Com Mcat Mcat Study Tips Mcat Study

/doctor-826e0c116cd549d98e327f1184c622d9.jpg)
